Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kobayashi, Keita; Nagai, Yuki; Itakura, Mitsuhiro; Shiga, Motoyuki
Journal of Chemical Physics, 155(3), p.034106_1 - 034106_9, 2021/07
Times Cited Count:5 Percentile:44.89(Chemistry, Physical)no abstracts in English
 C based on two binary non-ideal solid solutions
C based on two binary non-ideal solid solutionsWalker, C.; Suto, Shunkichi; Oda, Chie; Mihara, Morihiro; Honda, Akira
Cement and Concrete Research, 79, p.1 - 30, 2016/01
Times Cited Count:69 Percentile:90.65(Construction & Building Technology)Modeling the solubility behavior of calcium silicate hydrate (C-S-H) gel is important to make quantitative predictions of the degradation of hydrated ordinary Portland cement (OPC) based materials. Experimental C-S-H gel solubility data have been compiled from the literature, critically evaluated and supplemented with new data from the current study for molar Ca/Si ratios = 0.2-0.83. All these data have been used to derive a discrete solid phase (DSP) type C-S-H gel solubility model based on two binary non-ideal solid solutions in aqueous solution(SSAS). Features of the DSP type C-S-H gel solubility model include satisfactory predictions of pH values and Ca and Si concentrations for all molar Ca/Si ratios = 2.7  0 in the C-S-H system, portlandite (CH) for Ca/Si ratios
0 in the C-S-H system, portlandite (CH) for Ca/Si ratios  1.65, congruent dissolution at Ca/Si ratios = 0.85, and amorphous silica (SiO
1.65, congruent dissolution at Ca/Si ratios = 0.85, and amorphous silica (SiO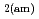 ) for Ca/Si ratios
) for Ca/Si ratios  0.55 as identified in the current study by IR spectroscopy.
0.55 as identified in the current study by IR spectroscopy.
Nishikawa, Hiroyuki*; Souno, T.*; Hattori, M.*; Oki, Y.*; Watanabe, E.*; Oikawa, Masakazu*; Arakawa, Kazuo; Kamiya, Tomihiro
JAERI-Review 2003-033, TIARA Annual Report 2002, p.254 - 256, 2003/11
no abstracts in English
Ozaki, Takuo; Ambe, Shizuko*; Abe, Tomoko*; Francis, A. J.
Biological Trace Element Research, 90(1-3), p.273 - 281, 2002/12
Times Cited Count:4 Percentile:6.56(Biochemistry & Molecular Biology)no abstracts in English
 with surface hydroxyl groups in lithium silicates
with surface hydroxyl groups in lithium silicatesNakazawa, Tetsuya; Yokoyama, Keiichi; Grismanovs, V.*; Katano, Yoshio*; Jitsukawa, Shiro
Journal of Nuclear Materials, 307-311(Part2), p.1436 - 1440, 2002/12
Times Cited Count:1 Percentile:10.15(Materials Science, Multidisciplinary)no abstracts in English
Nishikawa, Hiroyuki*; Souno, T.*; Hattori, M.*; Nishihara, Y.*; Oki, Y.*; Watanabe, E.*; Oikawa, Masakazu*; Kamiya, Tomihiro; Arakawa, Kazuo
Nuclear Instruments and Methods in Physics Research B, 191(1-4), p.342 - 345, 2002/05
Times Cited Count:3 Percentile:24.4(Instruments & Instrumentation)no abstracts in English
Nakazawa, Tetsuya; Yokoyama, Keiichi; Grismanovs, V.*; Katano, Yoshio*; Jitsukawa, Shiro
Journal of Nuclear Materials, 302(2-3), p.165 - 174, 2002/04
Times Cited Count:3 Percentile:23.41(Materials Science, Multidisciplinary)no abstracts in English
 O molecules from surface hydroxyl groups in silicates
O molecules from surface hydroxyl groups in silicatesNakazawa, Tetsuya; Yokoyama, Keiichi; Grismanovs, V.*; Katano, Yoshio*
Journal of Nuclear Materials, 297(1), p.69 - 76, 2001/07
Times Cited Count:9 Percentile:56.08(Materials Science, Multidisciplinary)no abstracts in English
Nakazawa, Tetsuya; Yokoyama, Keiichi; Grismanous, J.*; Katano, Yoshio*
Journal of Nuclear Materials, 279(2-3), p.201 - 206, 2000/06
Times Cited Count:10 Percentile:57.14(Materials Science, Multidisciplinary)no abstracts in English
Owada, Hitoshi*; Mihara, Morihiro; Iriya, Keishiro*; *
JNC TN8400 99-057, 43 Pages, 2000/03
Cementitious materials are considered as candidate materials for the geological disposal of high-level radioactive waste and TRU waste. As the pH and the Ca content of leachate from the cementitious materials are high, the host rock and the buffer-material would be degraded by the leachate in the long-term. Therefore, transport properties and parameters such as solubilities and distribution coefficients of radionuclides would be changed and affect the performance of the repository. In order to dissolve this "High pH plobrem", the use of a low alkalinity cement is considered for the disposal. In this study, we summarized the necessity of the low alkalinity cement, and developed the approach of the low alkalinization of cement. And, the following were carried out in this study : A leaching test of cement paste, a fluid test of the mortar and a installation test of the concrete to the trial structure. From the leaching test using the cement paste, we confirmed that we were able to obtain the low alkalinity cement (HFSC) by addition of pozzolanic materials such as silica-fume and flyash. From the result of the fluid test of the mortar, we chose the cement for the practicability evaluation. The practicability of low alkalinity concrete was evaluated by installation test to the trial structure.As a result of these examinations, we proved that the pH value of the leachate from the cementitious material was reduced by adding SF and FA to Portland cement. Simultaneously, SF and FA had to be added in order to obtain the good workability. In addition, workability and mechanical strength of the cement which SF and FA were added are almost equivalent to the ordinary Portland cement. The results shows that the HFSC has high practicability.
Tanaka, Satoru*
JNC TJ8400 2000-003, 62 Pages, 2000/02
For the safety assessment of radioactive waste disposal, it is important to elucidate the effect of colloids on radionuclide migration, which are released with dissolution of cementitious materials composing engineered barricr. In the previous work, we identified and characterized the colloidal particles in the solutions contacting cement hydrates, OPC and low-alkaline cement paste, and observed the release of the colloid particle. In the present work, we performed same experiments as the last year to confirm the reproducibility of the colloid release. We studied the leaching behavior of the colloid when OPC and low-alkaline cement past contact water flow. Furthermore, the effect of an alumina particle was studied, which is used as a barrier material for colloid migration. The following conclusions were derived: (1)In the solution contacting cement paste, the small amount of particles, which are considered as CaCO or silicate colloids were observed. Thus, the reproducibility of the last work was confirmed. (2)The leaching of colloid in the solution was confirmed by water flow through the cement paste. The concentration of particle was as low as 10
or silicate colloids were observed. Thus, the reproducibility of the last work was confirmed. (2)The leaching of colloid in the solution was confirmed by water flow through the cement paste. The concentration of particle was as low as 10
 10
10 mL
mL . (3)Al
. (3)Al 0
0 powder, with the diameter of 200
powder, with the diameter of 200 150
150 m, was found to be effective to some extent as a barrier for a colloid migration from low-alkaline cement paste.
m, was found to be effective to some extent as a barrier for a colloid migration from low-alkaline cement paste.
Kimura, Takaumi; Kato, Yoshiharu; Takeishi, Hideyo; Takahashi, Yoshio*; Minai, Yoshitaka*; Choppin, G. R.*
Proceedings of OECD/NEA Workshop on Evaluation of Speciation Technology, p.61 - 81, 1999/00
no abstracts in English
Futakawa, Masatoshi; Steinbrech, R. W.*
Journal of the American Ceramic Society, 81(7), p.1819 - 1842, 1998/07
no abstracts in English
Shikama, Tatsuo*; Kakuta, Tsunemi; *; Ishihara, Masahiro; ; Arai, Taketoshi
ECN-R--98-005, p.125 - 143, 1998/00
no abstracts in English
Uedono, Akira*; *; *; *; *; Ito, Hisayoshi
Journal of Physics; Condensed Matter, 7, p.5139 - 5149, 1995/00
Times Cited Count:10 Percentile:55.1(Physics, Condensed Matter)no abstracts in English
*; *; *; *; *; Suzuki, Junichi; Funahashi, Satoru
Langmuir, 11, p.563 - 567, 1995/00
Times Cited Count:36 Percentile:82.5(Chemistry, Multidisciplinary)no abstracts in English
Tanaka, Tadao; Yamamoto, Tadatoshi; *
Nihon Genshiryoku Gakkai-Shi, 37(1), p.51 - 58, 1995/00
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)no abstracts in English